Contact Changfu Chemical Now!
+86 27 8439 6550 | +86 181 6277 0058
An In-Depth Guide to Silylation Reagents: Applications and Benefits
Silylation reagents play a critical role in modern chemistry, with broad applications in organic synthesis, analytical chemistry, and materials science. These reagents have become indispensable in protecting reactive functional groups, enhancing reaction outcomes, and facilitating more precise analytical techniques. In this comprehensive guide, we will explore what silylation reagents are, how they work, their applications across various fields, and the benefits they bring to chemical processes.

What Are Silylation Reagents?
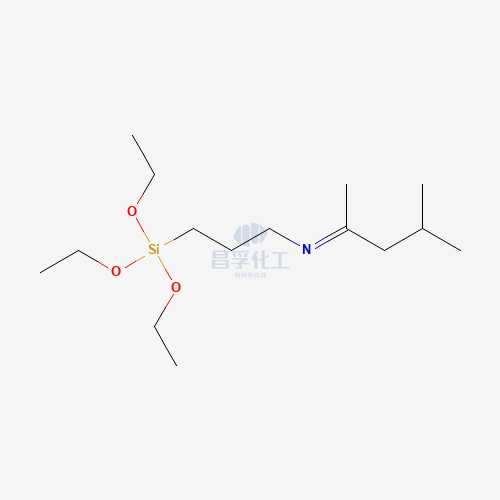
Silylation reagents are a class of chemical compounds used to introduce silyl groups (-SiR₃) into a molecule, often for the purpose of protecting reactive functional groups during chemical reactions. Silylation involves the substitution of a hydrogen atom from a hydroxyl, amino, or carboxyl group with a silyl group, commonly trimethylsilyl (TMS), leading to a more stable, less reactive intermediate.
These reagents are particularly important in organic synthesis and analytical techniques because they prevent unwanted side reactions and enhance the properties of the target compounds. Some of the most commonly used silylation reagents include trimethylsilyl chloride (TMS-Cl), hexamethyldisilazane (HMDS), and N,O-bis(trimethylsilyl)acetamide (BSA).
Mechanism of Silylation: How Does It Work?
The silylation process operates through a nucleophilic substitution mechanism, where a nucleophile—commonly a hydroxyl (-OH), amino (-NH₂), or carboxyl (-COOH) group—reacts with a silylation reagent. In this reaction, the nucleophile attacks the silicon atom of the reagent, which is electrophilic due to its polar Si-X bond (X being a halide or other leaving group). This leads to the replacement of a proton (H⁺) on the nucleophilic group by the silyl group (SiR₃), forming a silyl derivative.
For example, when a hydroxyl group reacts with trimethylsilyl chloride (TMS-Cl), the reaction yields a trimethylsilyl ether (R-O-Si(CH₃)₃), replacing the proton with a trimethylsilyl group. This conversion renders the functional group less reactive and more stable under various reaction conditions, allowing chemists to perform subsequent transformations without interference from the protected group. The process typically requires a base to neutralize any acidic by-products, such as HCl, formed during the reaction.
Types of Silylation Reagents
Silylation reagents can be classified based on the type of silyl group introduced and the target functional group. The most common silylation reagents include:
Trimethylsilyl (TMS) Reagents: TMS-Cl, TMS-Tf (trimethylsilyl triflate), and TMS-OTf (trimethylsilyl trifluoromethanesulfonate).
Dimethylsilyl (DMS) Reagents: Often used when steric hindrance is a concern.
Trialkylsilyl Reagents: For specific applications, other alkyl groups such as tert-butyldimethylsilyl (TBDMS) and triisopropylsilyl (TIPS) are used to provide additional stability or selectivity.
These reagents are chosen based on factors such as the stability of the resulting silyl ether and the reaction conditions required for the silylation process.
Applications of Silylation Reagents in Organic Chemistry
Protecting Functional Groups
One of the primary uses of silylation reagents in organic chemistry is to protect reactive functional groups, allowing for selective reactions elsewhere in the molecule. Functional groups such as hydroxyls (-OH), amines (-NH₂), and carboxyls (-COOH) can be highly reactive and prone to undesired side reactions. By temporarily converting these groups into silyl derivatives, chemists can perform other transformations without interference from these groups.
For example, during the synthesis of complex organic molecules, protecting the hydroxyl groups in a multi-step reaction is essential to prevent unwanted reactions with electrophiles or bases.
Enhancing Stability of Molecules
Silylation reagents enhance the stability of many organic compounds, particularly those that are sensitive to hydrolysis or oxidation. By replacing a reactive hydrogen atom with a silyl group, the molecule becomes less prone to degradation, enabling it to withstand harsh conditions.
Synthesis of Pharmaceuticals
In pharmaceutical synthesis, silylation is widely used for both protecting groups and as a part of the synthesis of drug molecules. For example, the introduction of silyl ethers can improve the solubility of certain compounds, making the synthesis more efficient. Silylation is also used in the preparation of chiral compounds and natural product synthesis, where selectivity and stability are critical.
Silylation in Analytical Chemistry
Gas Chromatography (GC)
One of the most common applications of silylation in analytical chemistry is in gas chromatography (GC). Silylation reagents are used to derivatize polar, non-volatile compounds, making them more volatile and suitable for GC analysis. By silylating functional groups like -OH and -COOH, the resulting compounds are more thermally stable and elute faster in GC columns, leading to better separation and analysis.
For example, in the analysis of fatty acids or alcohols by GC, these compounds are often silylated to increase volatility and improve detection sensitivity.
Mass Spectrometry (MS)
In mass spectrometry (MS), silylation can improve the ionization efficiency of polar compounds, leading to more accurate mass spectra. The increased volatility and reduced polarity of silylated compounds also make them easier to separate and identify in complex mixtures.
High-Performance Liquid Chromatography (HPLC)
Silylation reagents are used in HPLC to improve the separation and detection of compounds with hydroxyl or carboxyl groups. Silylating these groups reduces polarity and increases the retention time on non-polar columns, leading to better resolution.
Silylation in Materials Science
Surface Modification
In materials science, silylation is used to modify surfaces, particularly those of silica-based materials like glass and silicon wafers. Silylation agents can be applied to these surfaces to create hydrophobic coatings or to introduce functional groups that improve adhesion, stability, or reactivity. This is especially useful in the development of microelectronics, sensors, and nanomaterials.
Enhancing Properties of Silica-Based Materials
Silylation is also used to enhance the properties of silica-based materials in applications such as chromatography, where modified silica particles are used as stationary phases, and in catalysis, where silylated catalysts improve the efficiency and selectivity of reactions.
Benefits of Using Silylation Reagents
Increased Stability
One of the key benefits of silylation is the increased stability of the resulting silyl derivatives. Silylation reagents protect sensitive functional groups from degradation, oxidation, or hydrolysis, making it possible to conduct reactions under harsher conditions without compromising the integrity of the molecule.
Improved Yields
In organic synthesis, protecting groups introduced by silylation can significantly improve reaction yields by preventing unwanted side reactions. This is especially important in multi-step syntheses, where the purity and yield of intermediates are crucial for the overall success of the process.
Enhanced Selectivity
Silylation allows for selective reactions to occur at specific sites within a molecule. By protecting certain functional groups, chemists can direct the reaction to other parts of the molecule, improving the selectivity and overall efficiency of the process.
Choosing the Right Silylation Reagent: Factors to Consider
When selecting a silylation reagent, several factors should be taken into account:
Reactivity: The reagent should be reactive enough to quickly and efficiently silylate the target functional group.
Stability: The stability of the resulting silyl derivative should be suitable for the reaction conditions.
Solubility: The solubility of the reagent in the chosen solvent should be adequate to ensure a homogeneous reaction.
Steric Effects: Bulky silylation reagents can offer increased selectivity but may also require higher reaction times or temperatures.
Limitations and Challenges in Silylation
Despite its many advantages, silylation does have some limitations. One challenge is the reversibility of silylation under acidic or aqueous conditions, which can lead to the deprotection of functional groups before the desired reaction is complete. Additionally, the formation of by-products such as HCl can lead to unwanted side reactions, requiring careful control of reaction conditions and the use of bases or scavengers.
Future Trends in Silylation Technology
The development of more selective and efficient silylation reagents is an ongoing area of research. New reagents that offer improved stability, faster reaction times, and greater selectivity are continually being developed. Additionally, the application of silylation in new fields such as biotechnology and nanotechnology is opening up exciting possibilities for the future.
Conclusion
Silylation reagents are versatile tools in modern chemistry, with applications ranging from organic synthesis and analytical chemistry to materials science. Their ability to protect functional groups, improve stability, and enhance reaction outcomes makes them indispensable in both research and industry. Understanding how to choose and apply the right silylation reagent can significantly improve the efficiency and selectivity of chemical processes, offering numerous benefits across a wide range of fields.
By leveraging the power of silylation, chemists and researchers can overcome many of the challenges associated with reactive compounds, paving the way for more efficient and accurate chemical transformations.
Popular Silicon Compounds
Popular Silicon Compounds
Related News & Blog
Related News & Blog





































































































+86 27 8439 6550
+86 181 6277 0058
sales@cfsilanes.com
Optics Valley Bio-City
No. 666, Gaoxin Avenue
Hongshan District, Wuhan City

+86 27 8439 6550 | +86 181 6277 0058
sales@cfsilanes.com
Optics Valley Bio-City
No. 666, Gaoxin Avenue
Hongshan District, Wuhan City
Copyright © Hubei ChangFu Chemical Co., Ltd. All Rights